
Chemistry, 13.03.2021 01:00, mvtthewisdead
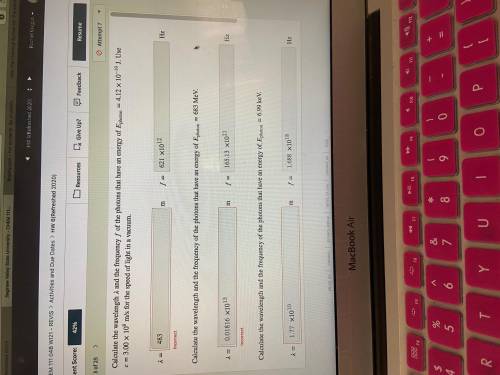
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J. Use =3.00×108 m/s for the speed of light in a vacuum.
Calculate the wavelength and the frequency of the photons that have an energy of photon=683 MeV.
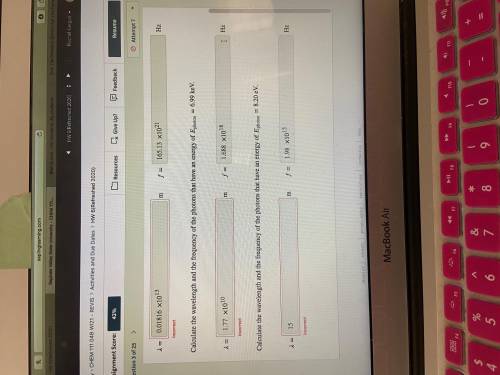
Calculate the wavelength and the frequency of the photons that have an energy of photon=6.99 keV.
Calculate the wavelength and the frequency of the photons that have an energy of photon=8.20 eV.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
Do you know the correct answer?
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J...
Questions in other subjects:
















