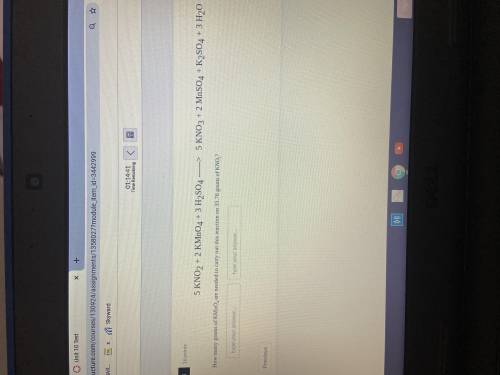
How many grams of KMnO4 are needed to carry out this reaction on 35.76 grams of KNO2
...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 23.06.2019 06:50, summerjoiner
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
Do you know the correct answer?
Questions in other subjects:






Biology, 08.01.2020 22:31




Mathematics, 08.01.2020 22:31