Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Do you know the correct answer?
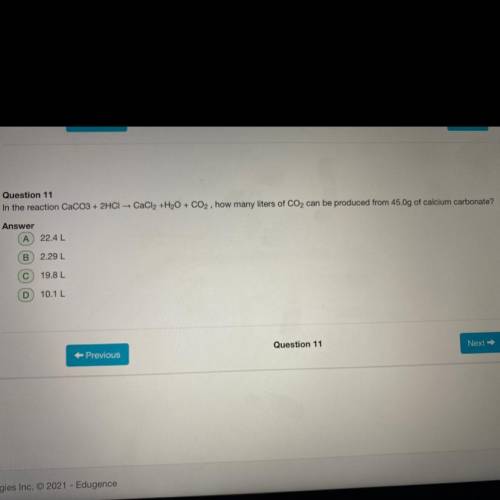
In the reaction CaCO3+2HCl CaCl 2 +H 2 O+CO 2 how many liters of CO 2 can be produced from 45.0g of...
Questions in other subjects:



Mathematics, 27.01.2020 01:31


Mathematics, 27.01.2020 01:31



Mathematics, 27.01.2020 01:31


Mathematics, 27.01.2020 01:31