
Chemistry, 12.03.2021 18:20, savannahvargas512
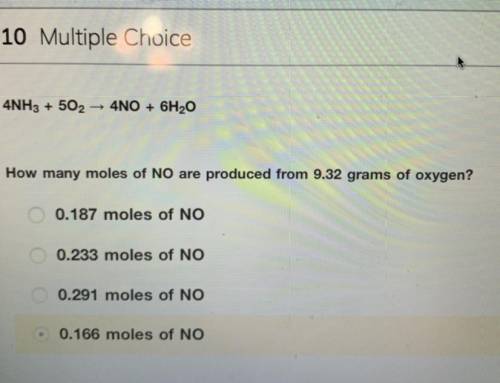
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
the answer is not 0.166 moles i tried and did not get credit for.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 10:30, jetblackcap
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
Do you know the correct answer?
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
Questions in other subjects:


English, 23.06.2019 23:00

Mathematics, 23.06.2019 23:00

Mathematics, 23.06.2019 23:00

Mathematics, 23.06.2019 23:00


Health, 23.06.2019 23:00








