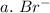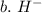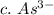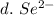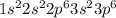a. fe

Chemistry, 02.02.2020 21:47, jazzycintron14
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
b. co
c. ni
2.write electron configurations for the 3 + 3 plus cations of these elements.
a. chromium
b. manganese
c. iron
3. write the symbol for the ion formed when each element gains electrons and attains a noble-gas electron configuration.
a. br
b. h
c. as
d. se
4.* write electron configurations for the following atoms and ions, and comment on the result.
ar
cl − cap cl to the minus
s 2 − cap s super 2 minus end super
p 3 −

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
Do you know the correct answer?
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
a. fe
Questions in other subjects:

Mathematics, 10.01.2022 14:00

Physics, 10.01.2022 14:00


Mathematics, 10.01.2022 14:00


Mathematics, 10.01.2022 14:00

Biology, 10.01.2022 14:00


Mathematics, 10.01.2022 14:00

Mathematics, 10.01.2022 14:00


![a.\ Fe^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s 3d^{5} \ or \ [Ar]4s 3d^{5}](/tpl/images/0494/0749/a44c8.png)
![b.\ Co^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{5} \ or \ [Ar]4s^{2} 3d^{5}](/tpl/images/0494/0749/d425b.png)
![c.\ Ni^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{6} \ or \ [Ar]4s^{2} 3d^{6}](/tpl/images/0494/0749/26975.png)
![a.\ Cr^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d \ or \ [Ar]4s^{2} 3d](/tpl/images/0494/0749/4de3e.png)
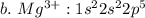
![c.\ Fe^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{3} \ or \ [Ar]4s^{2} 3d^{3}](/tpl/images/0494/0749/51e46.png)