
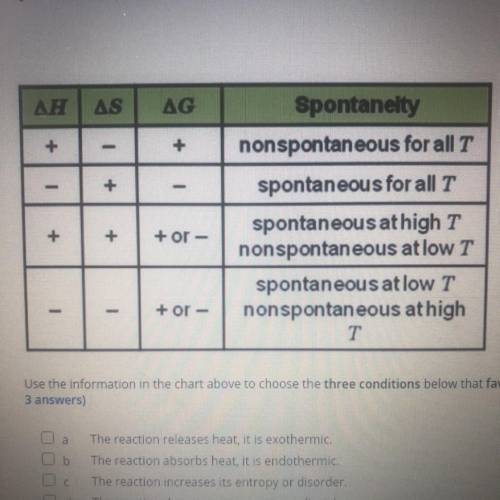
Can someone help me please
using the information in the chart above to choose the three conditions below that favor spontaneous reaction at all temperaturs ( Choose 3 answers)
A. The reaction release heat it is exothermic
B. The reaction absorbs heat it is endothermic
C. The reaction increases its entropy or disorder
D. The reaction decreases its entropy or disorder
E. The value of G is negative
F. The value of G is positive

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
Do you know the correct answer?
Can someone help me please
using the information in the chart above to choose the three conditions...
Questions in other subjects:

Mathematics, 22.08.2019 01:30





Mathematics, 22.08.2019 01:30


Mathematics, 22.08.2019 01:30

English, 22.08.2019 01:30







