
Chemistry, 11.03.2021 14:10, kaelynnmarie1135
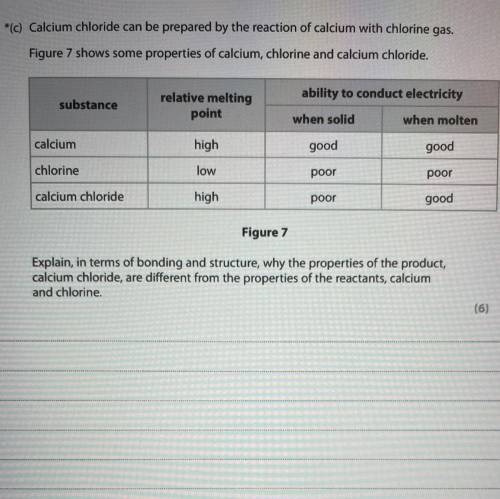
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
Figure 7 shows some properties of calcium, chlorine and calcium chloride.
ability to conduct electricity
substance
relative melting
point
when solid
when molten
calcium
high
good
good
chlorine
low
poor
poor
calcium chloride
high
poor
good
Figure 7
Explain, in terms of bonding and structure, why the properties of the product,
calcium chloride, are different from the properties of the reactants, calcium
and chlorine.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:00, andrejr0330jr
Which of the following is a chemical property of water at 4 c
Answers: 2
Do you know the correct answer?
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
Figure 7 shows some...
Questions in other subjects:





Mathematics, 30.10.2020 17:20










