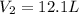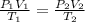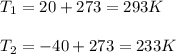A helium filled ballon had a volume of 8.50 L on the ground at 20.0 C and a pressure of 750.0 Torr. After the ballon was released, it rose to an altitude where the temperature was -40.0 C and the pressure was .550 atm. What is the new volume of the balloon in liters at the high altitude?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
Do you know the correct answer?
A helium filled ballon had a volume of 8.50 L on the ground at 20.0 C and a pressure of 750.0 Torr....
Questions in other subjects:



Mathematics, 20.04.2021 21:20

Mathematics, 20.04.2021 21:20

History, 20.04.2021 21:20