Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
Do you know the correct answer?
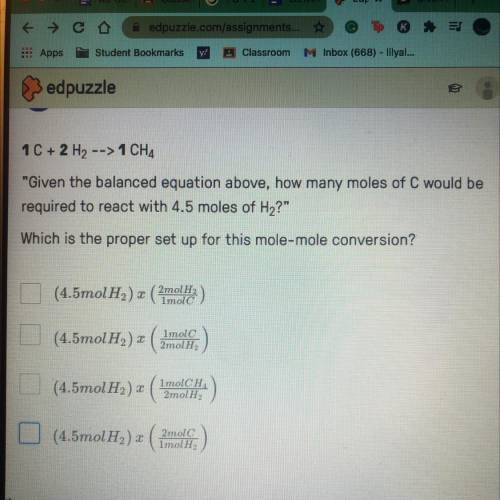
Given the balanced equation above, how many moles of C would be required to react with 4.5 moles of...
Questions in other subjects:


Mathematics, 29.10.2020 23:10

Mathematics, 29.10.2020 23:10



Mathematics, 29.10.2020 23:10

History, 29.10.2020 23:10


Advanced Placement (AP), 29.10.2020 23:10

Chemistry, 29.10.2020 23:10