
Chemistry, 11.03.2021 05:30, feliciagraham14
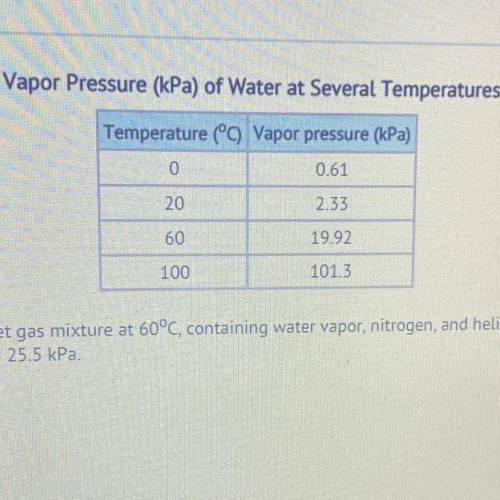
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and helium. The partial pressures are Pnitrogen = 53.0 kPa and Phelium = 25.5 kPa.
A
58.58 kPa
B)
78.50 kPa
C)
98.42 kPa
D
101.32 KP

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3
Do you know the correct answer?
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and heliu...
Questions in other subjects:

English, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00













