
Chemistry, 11.03.2021 02:20, osmarirodriguez2079
A chemical reaction in which hydrogen, H2, is combined with oxygen, O2, results in the production of the compound dihydrogen monoxide, H2O, commonly called water. What mass of water, to the nearest hundredth of a gram, is produced in this reaction? (Numbers ONLY)
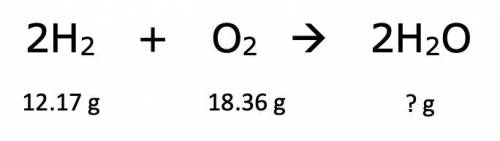

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
Do you know the correct answer?
A chemical reaction in which hydrogen, H2, is combined with oxygen, O2, results in the production of...
Questions in other subjects:


Health, 22.03.2021 03:00




English, 22.03.2021 03:00




Biology, 22.03.2021 03:00






