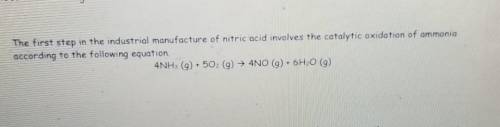How Many moles of oxygen(02) are needed to produce 4.6 g of nitrogen monoxide (NO)?
3.36 mol
...


Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 25.11.2021 14:00



History, 25.11.2021 14:00






Business, 25.11.2021 14:00