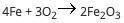Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1


Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation: (pict...
Questions in other subjects:

Mathematics, 05.11.2020 21:40

English, 05.11.2020 21:40

Chemistry, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40



Computers and Technology, 05.11.2020 21:40



Chemistry, 05.11.2020 21:40