2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
...

Chemistry, 10.03.2021 04:00, vrentadrienneoqug1a
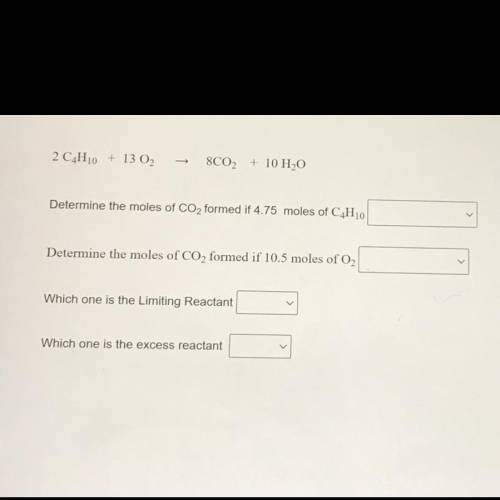
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
<
Determine the moles of CO2 formed if 10.5 moles of O2
Which one is the Limiting Reactant
Which one is the excess reactant

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1


Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Social Studies, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

History, 08.12.2020 01:00

Biology, 08.12.2020 01:00


English, 08.12.2020 01:00



Mathematics, 08.12.2020 01:00






