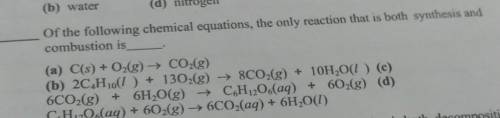(a) C(s) + O2(g) + CO2(g)
(b) 2C_Hjo(1 ) + 1302(g) → 8C02(g) + 10H2O(1)
(c) 6CO2(g) + 6H2O(g)...


Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
Questions in other subjects:





Mathematics, 28.10.2020 19:30

History, 28.10.2020 19:30

English, 28.10.2020 19:30

Health, 28.10.2020 19:30