
Chemistry, 09.03.2021 08:50, ashleyroberson735
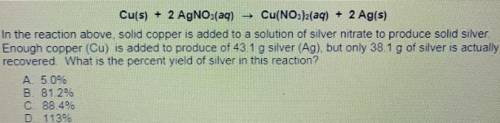
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a solution of silver nitrate to produce solid silver.
Enough copper (Cu) is added to produce of 431 g silver (Ag) but only 38.1 g of silver is actually
recovered What is the percent yield of silver in this reaction?
A. 5.0%
B. 81.2%
C 88 46
D. 1139

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1
Do you know the correct answer?
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a sol...
Questions in other subjects:

Mathematics, 28.01.2020 07:31


History, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

History, 28.01.2020 07:31




Geography, 28.01.2020 07:31

Chemistry, 28.01.2020 07:31






