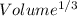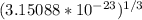Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid solution for all concentrations at room temperature. Compute the unit cell edge length for a 84 wt% W - 16 wt% Mo alloy. The room-temperature density and atomic weight of W are 19.3 g/cm3 and 183.85 g/mol, the room-temperature density and atomic weight of Mo are 10.22 g/cm3 and 95.94 g/mol, respectively.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1
Do you know the correct answer?
Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid sol...
Questions in other subjects:




Business, 10.12.2021 22:20

Mathematics, 10.12.2021 22:20



Mathematics, 10.12.2021 22:20

History, 10.12.2021 22:20