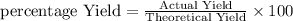Question 5
1 pts
What is the correct interpretation of an 85% yield for a given reactio...

Chemistry, 09.03.2021 04:10, lrich20200
Question 5
1 pts
What is the correct interpretation of an 85% yield for a given reaction?
85% of the anticipated amount of products were produced (relative to the theoretical yield)
O 15% of the reactants were used up
O 85% of the reactants were used up
15% of the anticipated amount of products were produced (relative to the theoretical yield)
Question 6
1 pts
True or false: The limiting reactant is the reactant that is used up after the reaction is complete.
O True
False

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Chemistry, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00




Mathematics, 27.04.2021 01:00