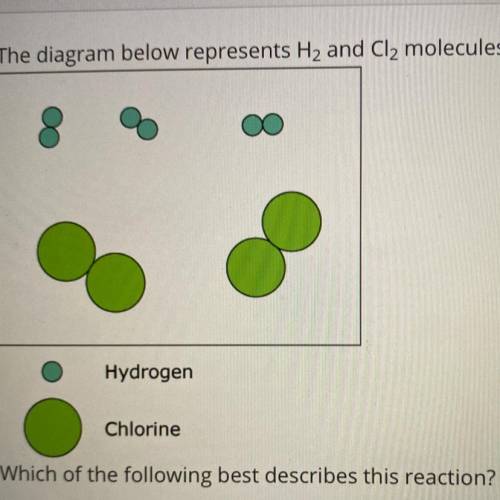
The diagram below represents H2 and Cl2 molecules that will potentially react to form HCI.
Hydrogen
Chlorine
Which of the following best describes this reaction?
А
The limiting reactant is chlorine.
B
The percent yield will be greater than 100%.
с
Hydrogen and chlorine are nonreactive.
D
All molecules shown will react to form the product.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, amariyanumber1923
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2


Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
Do you know the correct answer?
The diagram below represents H2 and Cl2 molecules that will potentially react to form HCI.
Hydrogen...
Questions in other subjects:

Mathematics, 05.05.2020 22:22


Mathematics, 05.05.2020 22:22



Chemistry, 05.05.2020 22:22