
Chemistry, 08.03.2021 21:40, samsavage4073
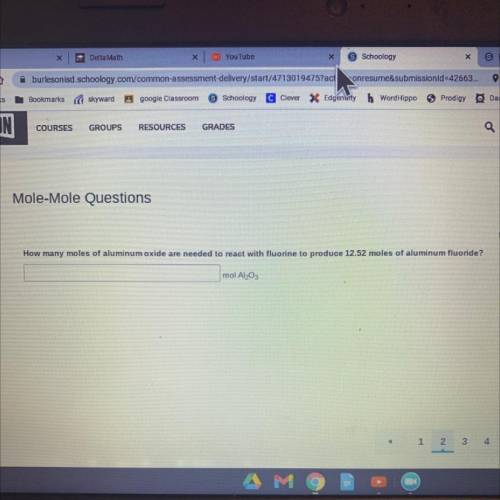
How many moles of aluminum oxide are needed to react with fluorine to produce 12.52 moles of aluminum fluoride? mol Al2O3

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2


Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
Do you know the correct answer?
How many moles of aluminum oxide are needed to react with fluorine to produce 12.52 moles of aluminu...
Questions in other subjects:

Mathematics, 04.11.2021 17:10



Mathematics, 04.11.2021 17:10

Mathematics, 04.11.2021 17:10


History, 04.11.2021 17:10

Computers and Technology, 04.11.2021 17:10

Social Studies, 04.11.2021 17:10

Mathematics, 04.11.2021 17:10






