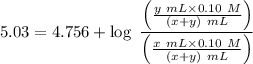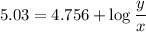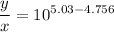Chemistry, 08.03.2021 20:50, tanviknawale
you have to prepare a ph 5.03 buffer, and you have the following 0.10m solutions available: hcooh, hcoona, ch3cooh, ch3coona, hcn, and nacn. How many milliliters of each solution would you use to make approximately a liter of the buffer?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
Do you know the correct answer?
you have to prepare a ph 5.03 buffer, and you have the following 0.10m solutions available: hcooh, h...
Questions in other subjects:


Mathematics, 19.12.2019 01:31





Mathematics, 19.12.2019 01:31

Biology, 19.12.2019 01:31

History, 19.12.2019 01:31

Mathematics, 19.12.2019 01:31


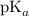 is nearly equal to the required pH) and the salt of its conjugate base.
is nearly equal to the required pH) and the salt of its conjugate base.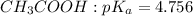
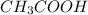 and
and 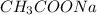 are x and y mL respectively.
are x and y mL respectively.![$pH = pK_a + \log \ \frac{[CH_3COONa]}{[CH_3COOH]}$](/tpl/images/1177/9578/5f8dc.png) .............(2)
.............(2)