Chemistry, 08.03.2021 20:10, alvaradovanessa14
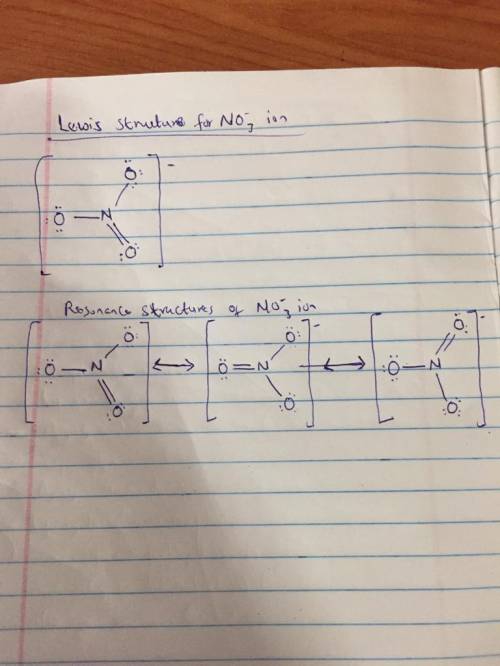
Discuss the nature of the bonding in the nitrate ion ( ) NO32 .Draw the possible Lewis resonance diagrams for this ion. Use the VSEPR theory to determine the steric number, thehybridization of the central N atom, and the geometry ofthe ion. Show how the use of resonance structures can beavoided by introducing a de-localized p MO. What bondorder is predicted by the MO model for the NUO bondsin the nitrate ion

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2
Do you know the correct answer?
Discuss the nature of the bonding in the nitrate ion ( ) NO32 .Draw the possible Lewis resonance dia...
Questions in other subjects:




Mathematics, 20.11.2020 15:40

Mathematics, 20.11.2020 15:40

Mathematics, 20.11.2020 15:40

Mathematics, 20.11.2020 15:40

Geography, 20.11.2020 15:40

Mathematics, 20.11.2020 15:40

Mathematics, 20.11.2020 15:40