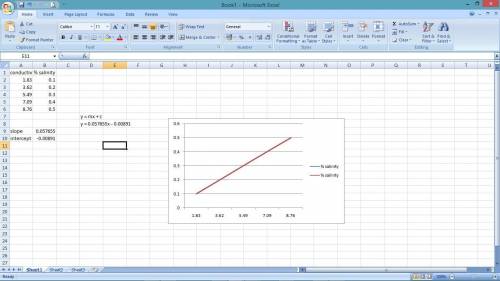
In chemistry lab student is determining the salinity of seawater using a conductivity meter. The student made standards of NaCl solutions and recorded the following data:
% Salinity Conductivity (ms)
0.10 1.83
0.20 3.62
0.30 5.49
0.40 7.09
0.50 8.76
The conductance of a 10 mL sample of unknown was found to be 5.82 ms.
Required:
a. Prepare a standard curve using the data above.
b. Determine the concentration of the unknown salt solution using the standard curve.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 23.06.2019 12:50, jasonoliva13
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
Do you know the correct answer?
In chemistry lab student is determining the salinity of seawater using a conductivity meter. The stu...
Questions in other subjects:

Chemistry, 08.10.2019 18:00