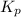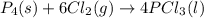Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1


Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
Do you know the correct answer?
Equilibrium constant Kp for the reaction P4(s) + 6Cl2(g) 4PCl3(l) Kp = 1/ P6Cl2 Kp = P4Cl3/ P6P4 Kp...
Questions in other subjects:


Mathematics, 12.12.2020 17:00


History, 12.12.2020 17:00


Social Studies, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Biology, 12.12.2020 17:00

Computers and Technology, 12.12.2020 17:00


![K_p=\frac{1}{[p_{Cl_2}]^6}](/tpl/images/1177/2723/ab503.png)