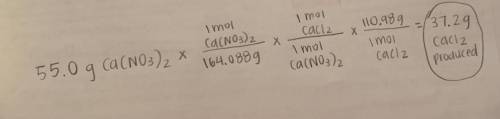Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
Do you know the correct answer?
Ca(NO3)2 + HCl --> HNO3 + CaCl2
According to the reaction above, 55.0 grams of calcium nitrate r...
Questions in other subjects:

Biology, 13.10.2020 21:01





Mathematics, 13.10.2020 21:01


English, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

History, 13.10.2020 21:01