This
Part A
A sample of ideal gas at room temperature occupies a volume of 21.0 L at a pressu...

Chemistry, 06.03.2021 22:10, thomascoop85
This
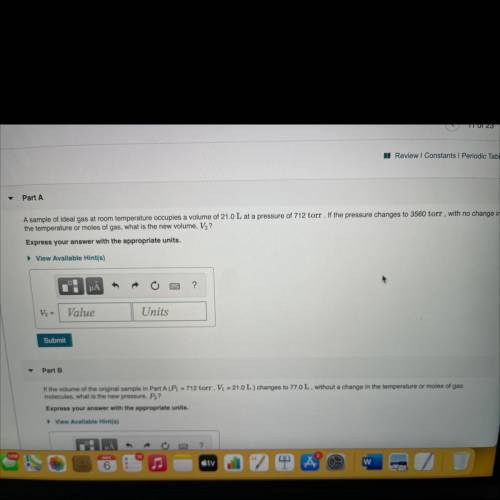
Part A
A sample of ideal gas at room temperature occupies a volume of 21.0 L at a pressure of 712 torr. If the pressure changes to 3560 torr , with no change in
the temperature or moles of gas, what is the new volume, V?
Express your answer with the appropriate units.
View Available Hint(s)
HÅ
?
of 1
V2 =
Value
Units
Submit

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 29.09.2020 06:01

Chemistry, 29.09.2020 06:01


History, 29.09.2020 06:01



History, 29.09.2020 06:01






