
Chemistry, 06.03.2021 01:00, alecnewman2002
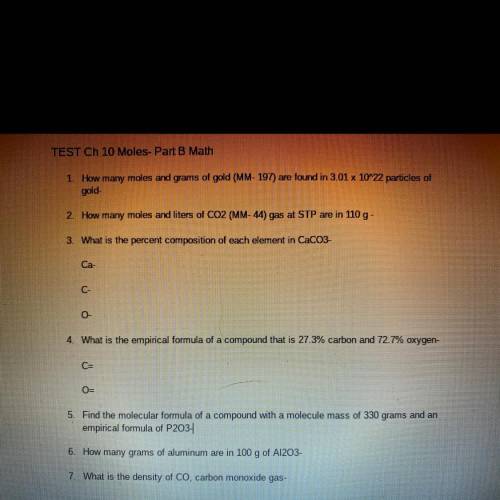
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^22 particles of
gold-
2. How many moles and liters of CO2 (MM-44) gas at STP are in 110 g -
3. What is the percent composition of each element in CaCO3-
Ca-
C-
0
4. What is the empirical formula of a compound that is 27.3% carbon and 72.7% oxygen-
C=
O-
5. Find the molecular formula of a compound with a molecule mass of 330 grams and an
empirical formula of P203-
6. How many grams of aluminum are in 100 g of A1203-
7. What is the density of Co, carbon monoxide gas-

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3


Chemistry, 23.06.2019 12:30, okasiafolk27
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
Do you know the correct answer?
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^...
Questions in other subjects:















