Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
Do you know the correct answer?
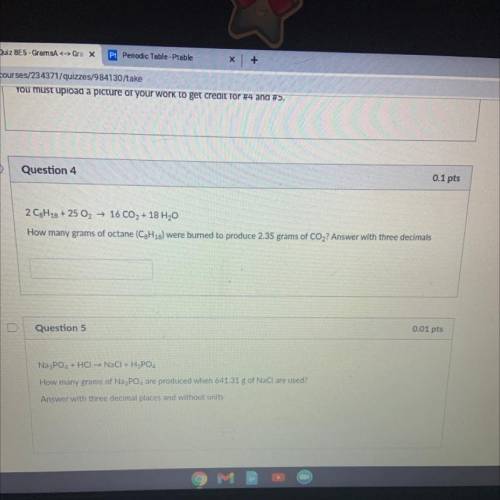
2 C8H18 + 25 O2 → 16 CO2 + 18 H20
How many grams of octane (C3H18) were burned to produce 2.35 gram...
Questions in other subjects:

Social Studies, 09.10.2019 14:50

Physics, 09.10.2019 14:50




Mathematics, 09.10.2019 14:50

Physics, 09.10.2019 14:50

History, 09.10.2019 14:50

Mathematics, 09.10.2019 14:50