
Chemistry, 05.03.2021 17:10, penaceci15
Look at the following image and explain why the row of storms is expected in the middle of the map.
A. Storms are expected due to high-pressure and low-pressure fronts.
B. Storms are expected due to the low-pressure system.
C. Storms are expected due to the high-pressure system.
D. Storms are expected due to the separation of the high and low pressure system.
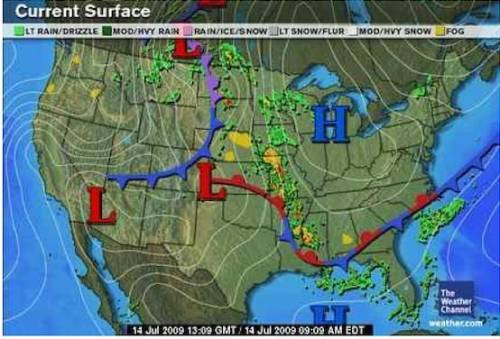

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
Do you know the correct answer?
Look at the following image and explain why the row of storms is expected in the middle of the map....
Questions in other subjects:



Mathematics, 24.12.2019 01:31


Biology, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31



Mathematics, 24.12.2019 01:31






