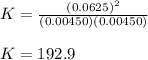Chemistry, 05.03.2021 14:00, hoopstarw4438
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. Determine the balanced equation and write the equilibrium expression
b. Determine the K eq
c. Will this process favor the reactants or products at equilibrium?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Do you know the correct answer?
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. De...
Questions in other subjects:

Mathematics, 26.03.2021 23:00

Mathematics, 26.03.2021 23:00

Mathematics, 26.03.2021 23:00



English, 26.03.2021 23:00


Health, 26.03.2021 23:00

Mathematics, 26.03.2021 23:00

Mathematics, 26.03.2021 23:00



![K=\frac{[HCl]^2}{[H_2][Cl_2]}](/tpl/images/1171/4277/48ea9.png)