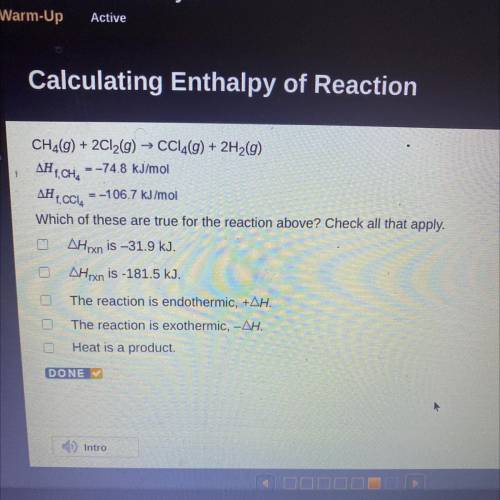
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
...

Chemistry, 05.03.2021 07:00, HaydenSturgis1
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
Which of these are true for the reaction above? Check all that apply.
AHrxn is –31.9 kJ.
AHrxn is -181.5 kJ.
The reaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a product.
PLEASE HELP IM BEGGING AND I WILL GIVE BRAINLIEST

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
Do you know the correct answer?
Questions in other subjects:







Mathematics, 27.04.2021 23:20



Mathematics, 27.04.2021 23:20






