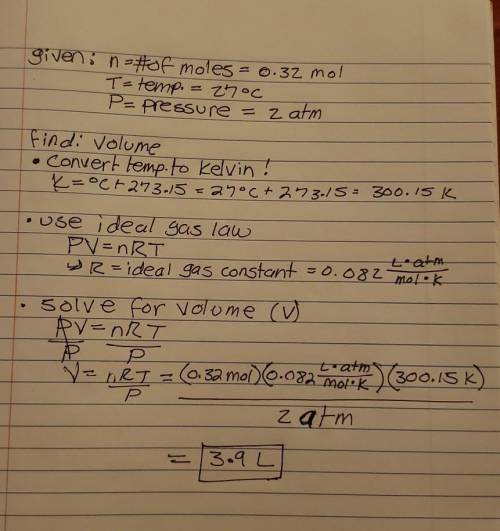Chemistry, 05.03.2021 06:20, bailey1025
0.32 moles of oxygen gas has a temperature of 27°C and pressure of 2 atm in a closed container. What is the volume?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
0.32 moles of oxygen gas has a temperature of 27°C and pressure of 2 atm in a closed container. What...
Questions in other subjects:




Business, 08.12.2021 18:00

SAT, 08.12.2021 18:00

Physics, 08.12.2021 18:00




English, 08.12.2021 18:00