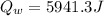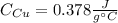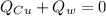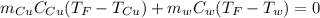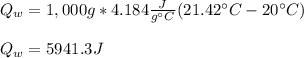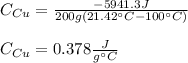Chemistry, 05.03.2021 04:30, ibrahimuskalel
Mix 200 g of copper at 100 °C with 1,000 g of water at 20 °C. Final temp. = 21.42°C a) How much heat energy (q) did the water gain? b) Now solve for the specific heat (c) of copper:

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
Do you know the correct answer?
Mix 200 g of copper at 100 °C with 1,000 g of water at 20 °C. Final temp. = 21.42°C a) How much heat...
Questions in other subjects:

Mathematics, 02.09.2021 20:30


Mathematics, 02.09.2021 20:30

Mathematics, 02.09.2021 20:30






Physics, 02.09.2021 20:30