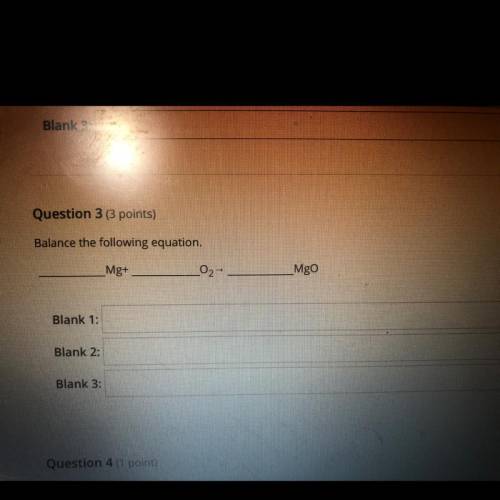
Balance the following equation.
Mg+
02-
Mgo
Blank 1:
Blank 2:
Blank 3...

Chemistry, 05.03.2021 01:00, Tyrant4life
Balance the following equation.
Mg+
02-
Mgo
Blank 1:
Blank 2:
Blank 3:
Question 4 (1 point)
This equation is balanced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Mathematics, 06.04.2022 22:40

Mathematics, 06.04.2022 22:40

Mathematics, 06.04.2022 22:40

English, 06.04.2022 22:40


Social Studies, 06.04.2022 23:00







