Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
Do you know the correct answer?
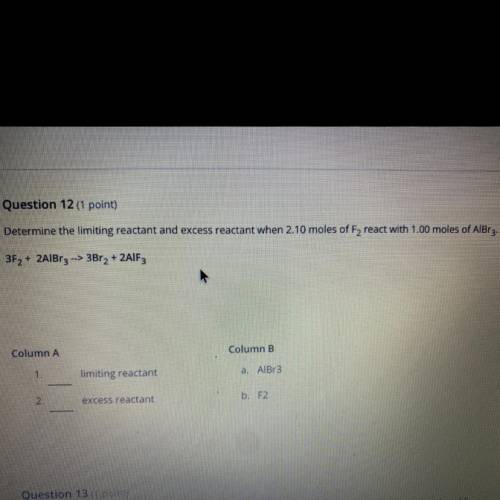
Question 12 (1 point)
Determine the limiting reactant and excess reactant when 2.10 moles of F, rea...
Questions in other subjects:



Mathematics, 27.10.2020 19:40


English, 27.10.2020 19:40

Mathematics, 27.10.2020 19:40

History, 27.10.2020 19:40

Mathematics, 27.10.2020 19:40


Chemistry, 27.10.2020 19:40