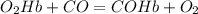Chemistry, 18.12.2019 23:31, shinekamui
According to the chemical reaction, if there are 4.5 mol of oxygenated hemoglobin present in an excess of carbon monoxide, how many moles of hemoglobin would release oxygen and bind to carbon monoxide? explain your answer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, ddmoorehouseov75lc
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2



Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
Do you know the correct answer?
According to the chemical reaction, if there are 4.5 mol of oxygenated hemoglobin present in an exce...
Questions in other subjects:

Mathematics, 03.06.2020 13:00


Mathematics, 03.06.2020 13:00

Mathematics, 03.06.2020 13:00


Law, 03.06.2020 13:00




Social Studies, 03.06.2020 13:00