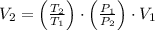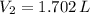Chemistry, 04.03.2021 22:00, yfgkeyonna
a 2.7 L of N2 is collected at 121kpa and 288 K . if the pressure increases to 202 kpa and the temperature rises to 303 K , what volume will the gas occupy?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 08:00, mackaylabarnes22
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
Do you know the correct answer?
a 2.7 L of N2 is collected at 121kpa and 288 K . if the pressure increases to 202 kpa and the temper...
Questions in other subjects:

Mathematics, 29.06.2019 03:50





Geography, 29.06.2019 03:50

Geography, 29.06.2019 03:50

Biology, 29.06.2019 03:50


Biology, 29.06.2019 03:50


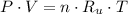 (1)
(1)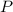 - Pressure, measured in kilopascals.
- Pressure, measured in kilopascals.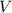 - Volume, measured in liters.
- Volume, measured in liters.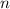 - Molar quantity, measured in moles.
- Molar quantity, measured in moles.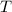 - Temperature, measured in Kelvin.
- Temperature, measured in Kelvin.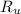 - Ideal gas constant, measured in kilopascal-liters per mole-Kelvin.
- Ideal gas constant, measured in kilopascal-liters per mole-Kelvin.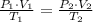 (2)
(2)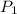 ,
, 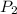 - Initial and final pressure, measured in kilopascals.
- Initial and final pressure, measured in kilopascals.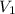 ,
, 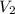 - Initial and final volume, measured in liters.
- Initial and final volume, measured in liters.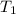 ,
, 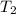 - Initial and final temperature, measured in Kelvin.
- Initial and final temperature, measured in Kelvin.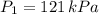 ,
, 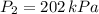 ,
, 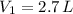 ,
, 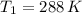 and
and 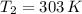 , the final volume of the gas is:
, the final volume of the gas is: