
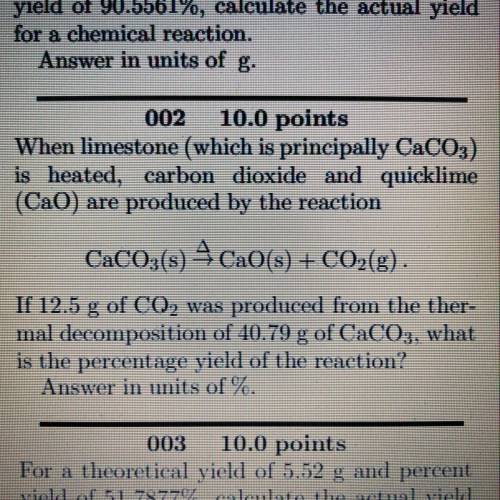
When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are produced by the reaction
CaCO3(s) 4CaO(s) + CO2(g).
If 12.5 g of CO2 was produced from the ther-
mal decomposition of 40.79 g of CaCO3, what
is the percentage yield of the reaction?
Answer in units of %.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are...
(Cao) are...
Questions in other subjects:

English, 27.01.2021 01:00

Biology, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


English, 27.01.2021 01:00







