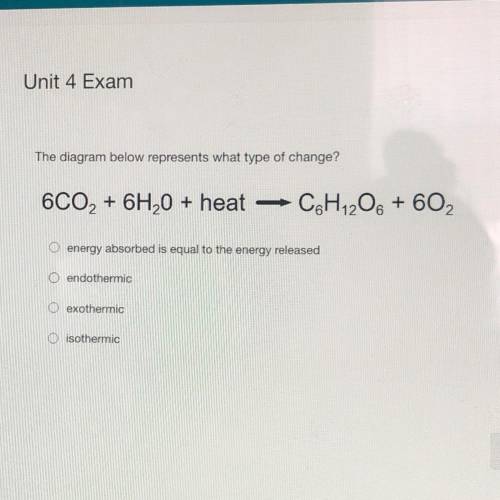
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
...

Chemistry, 04.03.2021 19:50, SoccerHalo
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
O energy absorbed is equal to the energy released
O endothermic
exothermic
O isothermic

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
Questions in other subjects:

English, 11.07.2019 09:50

Biology, 11.07.2019 09:50


Spanish, 11.07.2019 09:50

Mathematics, 11.07.2019 09:50



History, 11.07.2019 09:50







