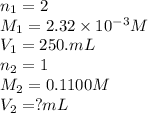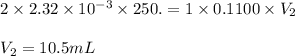Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml...

Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1100m naoh solution.
calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Do you know the correct answer?
Questions in other subjects:



Mathematics, 14.01.2021 02:50

English, 14.01.2021 02:50


Mathematics, 14.01.2021 02:50

Mathematics, 14.01.2021 02:50



Physics, 14.01.2021 02:50



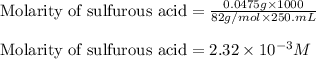
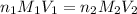
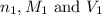 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 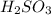
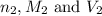 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.