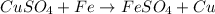Chemistry, 04.03.2021 09:00, francisco42002
(a)A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
(i) What type of colour change do you observe?
(ii) Identify the type of reaction.
(iii) Write a word equation for the above change.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3


Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
Do you know the correct answer?
(a)A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and le...
Questions in other subjects:

English, 09.04.2021 21:20


Social Studies, 09.04.2021 21:20


Mathematics, 09.04.2021 21:20


Mathematics, 09.04.2021 21:20


Mathematics, 09.04.2021 21:20

Social Studies, 09.04.2021 21:20


 iron sulphate + copper
iron sulphate + copper