
Chemistry, 03.03.2021 21:20, micahwilkerson9495
Please help! Very important. Brainliest to correct.
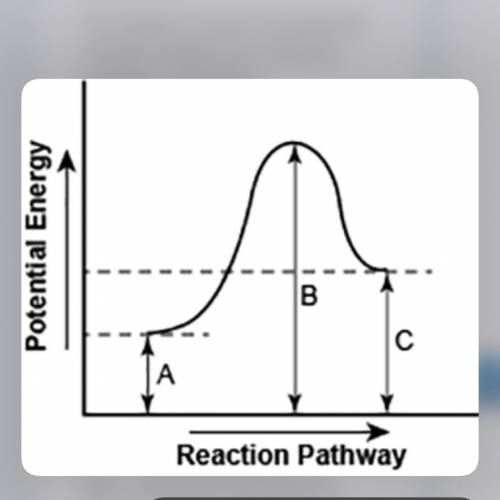
The diagram shows the potential energy changes for a reaction pathway. (10 points)
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1


Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1
Do you know the correct answer?
Please help! Very important. Brainliest to correct.
The diagram shows the potential energy changes...
Questions in other subjects:


Chemistry, 18.05.2021 18:50







Mathematics, 18.05.2021 18:50






