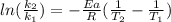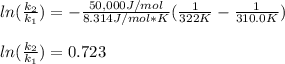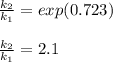Chemistry, 03.03.2021 08:50, tristan4233
The activation energy Ea for a particular reaction is 50.0 kJ/mol. How
much faster is the reaction at 322 K than at 310.0 K? (R = 8.314 J/mol
•K)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1
Do you know the correct answer?
The activation energy Ea for a particular reaction is 50.0 kJ/mol. How
much faster is the reaction...
Questions in other subjects:





Mathematics, 10.12.2019 10:31


English, 10.12.2019 10:31


History, 10.12.2019 10:31

History, 10.12.2019 10:31