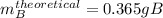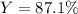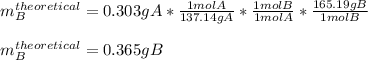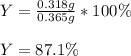Chemistry, 03.03.2021 08:30, gamerdoesart
PLEASE HELP
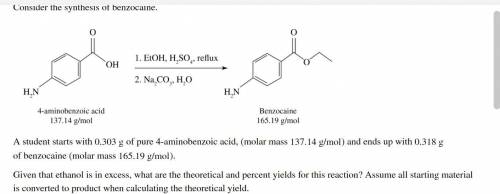
A student starts with 0.303 g of pure 4-aminobenzoic acid, (molar mass 137.14 g/mol) and ends up with 0.318 g of benzocaine (molar mass 165.19 g/mol).
Given that ethanol is in excess, what are the theoretical and percent yields for this reaction? Assume all starting material is converted to product when calculating the theoretical yield

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, juandavidklingera553
What would you do if you told the guy you liked that you liked him
Answers: 1


Do you know the correct answer?
PLEASE HELP
A student starts with 0.303 g of pure 4-aminobenzoic acid, (molar mass 137.14 g/mol) an...
Questions in other subjects:

Mathematics, 09.02.2022 09:30

Mathematics, 09.02.2022 09:30





Social Studies, 09.02.2022 09:30



Chemistry, 09.02.2022 09:30