
Chemistry, 03.03.2021 01:40, trentonmccary2096
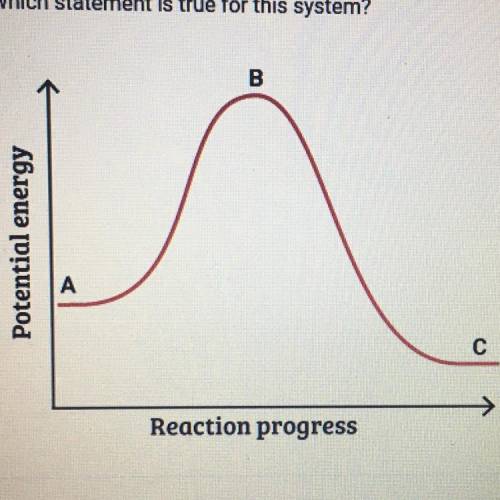
This graph shows how the potential energy of a reaction system changes
over time. Which statement is true for this system
A. The potential energy of the reactants is greater than the potential
energy of the products.
B. The height of the curve at point A represents the activation energy.
C. The height of the curve at point B represents the activation energy.
D. The potential energy of the products is greater than the potential
energy of the reactants.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
Do you know the correct answer?
This graph shows how the potential energy of a reaction system changes
over time. Which statement i...
Questions in other subjects:




Mathematics, 15.12.2021 07:40


History, 15.12.2021 07:50

Mathematics, 15.12.2021 07:50


Mathematics, 15.12.2021 07:50

Mathematics, 15.12.2021 07:50






