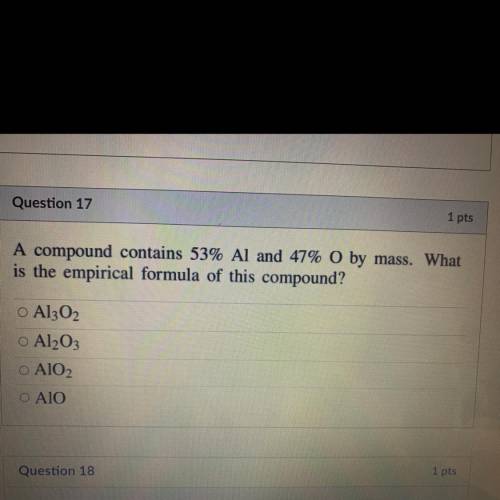
A compound contains 53% Al and 47% O by mass. What
is the empirical formula of this compound?...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1


Chemistry, 23.06.2019 22:00, johnisawesome999
Sugar will melt ice but is not as effective as salt because salt breaks down into sodium and chloride ions so when one molecule of salt dissolves into the ice, it will add two components to the solution which provide more interference in preventing the water molecules from freezing.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

English, 23.06.2021 21:40


Mathematics, 23.06.2021 21:40



Mathematics, 23.06.2021 21:50

Mathematics, 23.06.2021 21:50

Mathematics, 23.06.2021 21:50

Physics, 23.06.2021 21:50

Mathematics, 23.06.2021 21:50