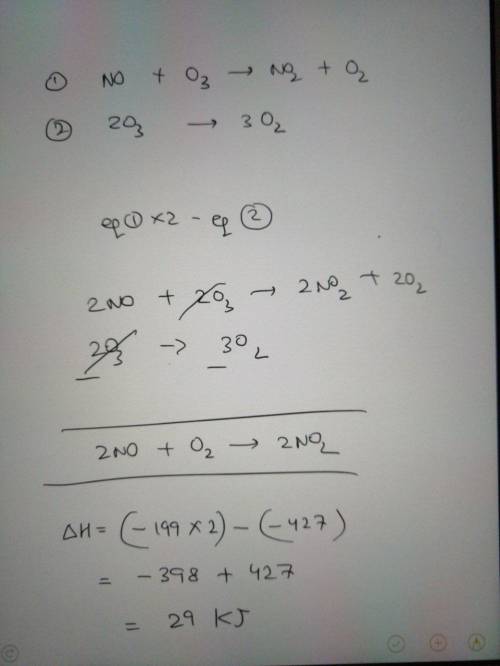Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO...

Chemistry, 02.03.2021 14:40, dinosaur10
Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO(g) + O2(g) → 2 NO2(g)
Determine the enthalpy change for this reaction
using any of these thermochemical equations:
02(g) →20(g)
AH = +495 kJ

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 15.04.2021 06:40

Mathematics, 15.04.2021 06:40

Medicine, 15.04.2021 06:40


Health, 15.04.2021 06:40

Geography, 15.04.2021 06:40

Biology, 15.04.2021 06:40

History, 15.04.2021 06:40