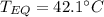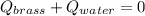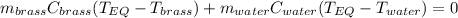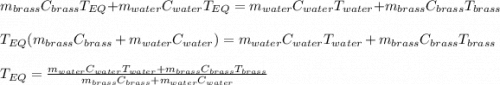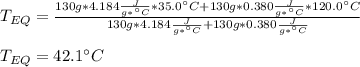Chemistry, 02.03.2021 14:00, LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 10:00, isaiahromero15
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 11:30, angelicar1160
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
Do you know the correct answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions in other subjects:

Social Studies, 27.11.2019 13:31

Social Studies, 27.11.2019 13:31

Mathematics, 27.11.2019 13:31

English, 27.11.2019 13:31


Social Studies, 27.11.2019 13:31

English, 27.11.2019 13:31

Social Studies, 27.11.2019 13:31

Social Studies, 27.11.2019 13:31

History, 27.11.2019 13:31