
Chemistry, 02.03.2021 14:00, mathman783
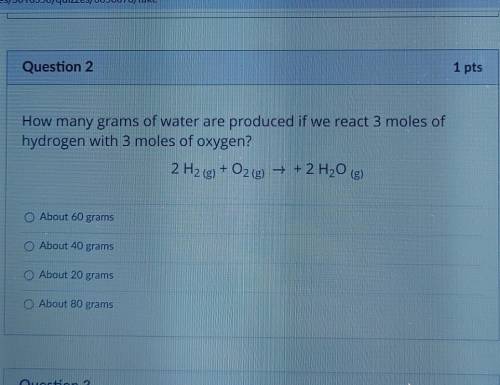
How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen?
About 60 grams
About 40 grams
About 20 grams
About 80 grams

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
Do you know the correct answer?
How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen?
About...
Questions in other subjects:

History, 16.10.2020 05:01


Chemistry, 16.10.2020 05:01


Social Studies, 16.10.2020 05:01




Geography, 16.10.2020 05:01






