Chemistry, 01.03.2021 23:10, harveyangel123p2tjae
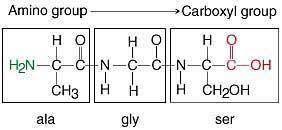
Examine the peptide. Peptide with three amino acid residues. The first residue contains a methyl group side chain. The second residue contains a hydrogen atom side chain. The third residue contains a hydroxymethyl group side chain. The amino group of the first side chain binds to the unshown remainder of the peptide. The carboxyl group of the third side chain binds to the unshown remainder of the peptide. Determine which amino acids are present in the peptide.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
Examine the peptide. Peptide with three amino acid residues. The first residue contains a methyl gro...
Questions in other subjects:









English, 13.07.2021 15:40